|
Problem? – No Problem!
At the first look one might think that Sodium
Borohydride as a fuel for Fuel Cells or for Hydrogen generation is
unpractical and expensive for the consumer. As often that first look
just scratches the surface of the topic and does not pointed towards
the future. Remember: Hydrogen and Fuel Cells are new territories
and no world standard is yet set. As soon as it comes to mass
production prices equal to the oil industry can and will be
achieved.
In the following text you can see and read
about, how easy and inexpensive the production- and recycling/
reproduction process of Sodium Borohydride is.
New Patented Processes of NaBH4
Production by MERIT Ltd.
With these new processes MERIT Ltd. has
radically lowered the price and the time for the
production/reproduction of Sodium Borohydride.
- Powder shape reactants are used.
- Non-hydrogenated alkaline metal (Mg) issued
as reactant.
- Heating treatment under reaction can increase
the reaction speed.
- Minimum energy input for reaction is
required.
Scheme of Dynamic hydriding/dehydriding
process
NaBO2 + 2Mg + 2H2 ü©
NaBH4 + 2MgO
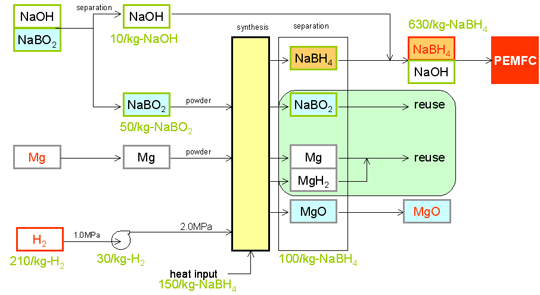
Material cost vs. commercial
process
* Note: The efficiency of the commercial
process by Bayer is about the same as the one of MERIT Ltd. One big
difference between those two processes is the price of the reducing
agent.
|